PSORIAPALM NON-GREASY PSORIASIS - salicylic acid lotion
Neopharm Co., Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
PSORIAPALM Non-Greasy Psoriasis Lotion
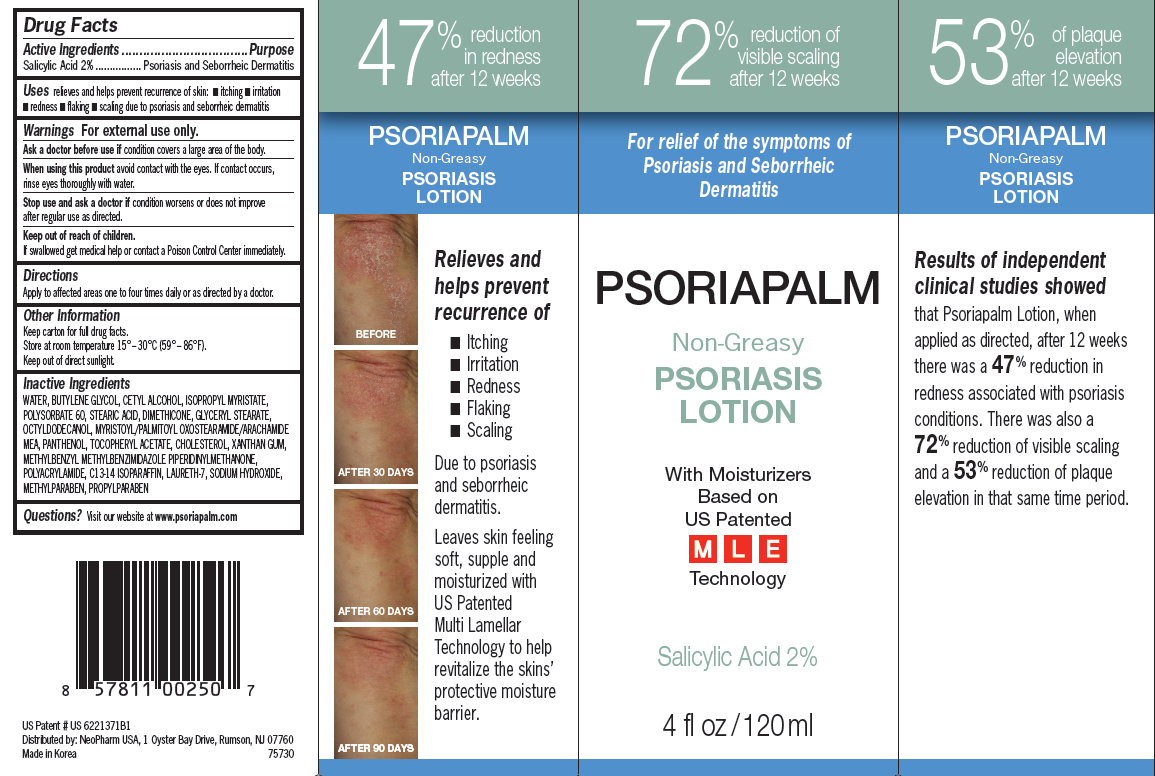
Drug Facts
Active ingredient
Salicylic Acid 2%
Purpose
Psoriasis and Seborrheic Dermatitis
Uses
relieves and helps prevent recurrence of skin:
- itching
- irritation
- redness
- flaking
- scaling
due to psoriasis and seborrheic dermatitis
Warnings
For external use only
Ask a doctor before use if condition covers a large area of the body.
When using this product avoid contact with the eyes. If contact occurs, rinse the eyes thoroughly with water.
Stop use and ask a doctor if condition worsens or does not improve after regular use as directed
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
Apply to affected areas one to four times daily or as directed by a doctor
Other Information
Store at room temperature 15° - 30°C (59° - 86°F). Keep out of direct sunlight.
Inactive ingredients
WATER, BUTYLENE GLYCOL, CETYL ALCOHOL, ISOPROPYL MYRISTATE, POLYSORBATE 60, STEARIC ACID, DIMETHICONE, GLYCERYL STEARATE, OCTYLDODECANOL, MYRISTOYL/PALMITOYL OXOSTEARAMIDE/ARACHAMIDE
MEA, PANTHENOL, TOCOPHERYL ACETATE, CHOLESTEROL, XANTHAN GUM, METHYLBENZYL METHYLBENZIMIDAZOLE PIPERIDINYLMETHANONE, POLYACRYLAMIDE, C13-14 ISOPARAFFIN, LAURETH-7, SODIUM HYDROXIDE,
METHYLPARABEN, PROPYLPARABEN
Questions?
Visit our website at www.psoriapalm.com
Distributed by:
Neopharm USA, 1 Oyster Bay Drive, Rumson, NJ 07760
Made in Korea
PSORIAPALM Non-Greasy Psoriasis Lotion 4oz (51141-8000-4)